Effect of silane coupling agent on properties of silicone co
By controlling the content of curing agent and silane coupling agent in the formulation of silicone coating, the elastic modulus, hardness, hydrophobicity, water absorption and water resistance of the coating materials were studied
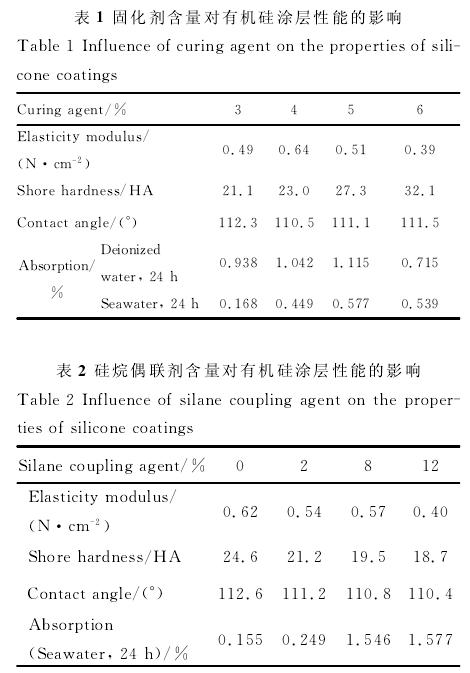
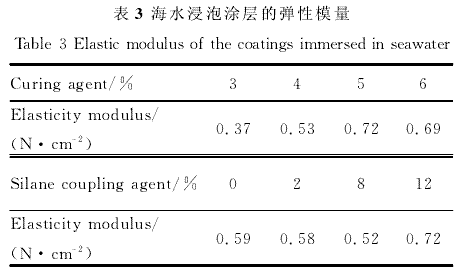
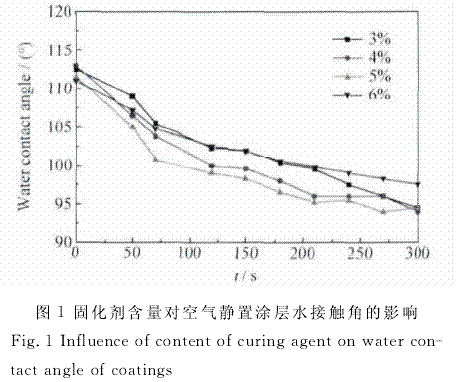
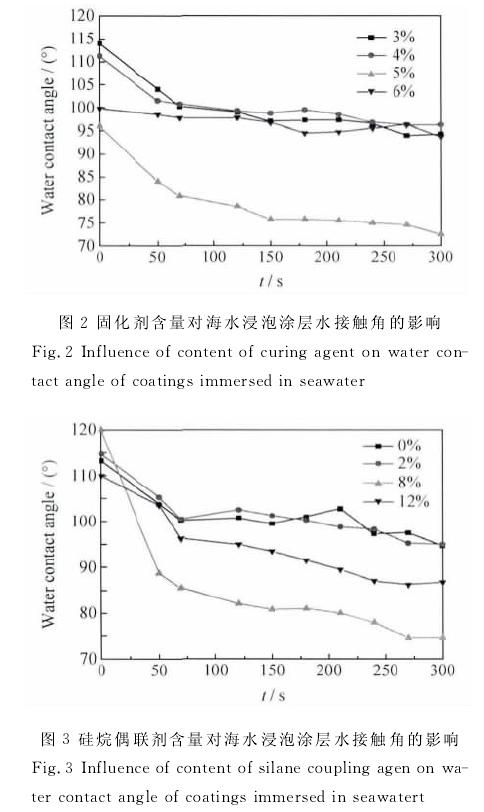
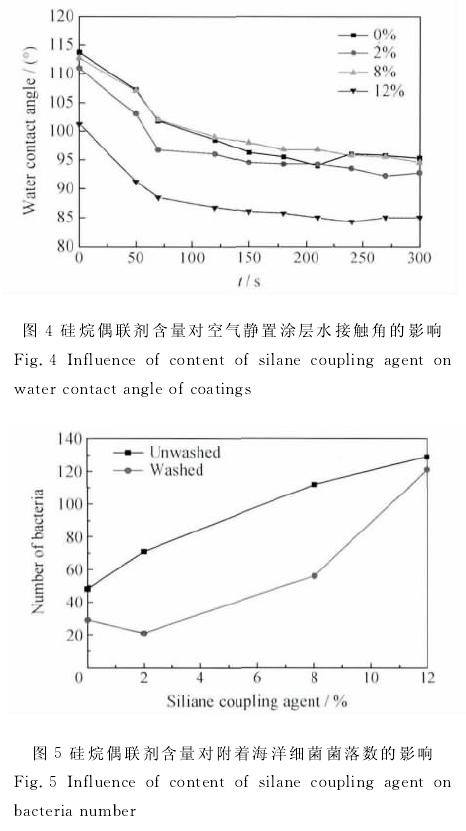
By controlling the content of curing agent and silane coupling agent in silicone coating formulation, the effects of curing agent and silane coupling agent on the elastic modulus, hardness, hydrophobicity, water absorption and water resistance of coating materials were studied. The results show that when the content of curing agent is more than 4%, with the increase of the content of curing agent, the elastic modulus of the coating decreases, the shore hardness increases, and the water absorption increases; with the increase of the content of silane coupling agent, the elastic modulus and water absorption of the silicone coating increases, while the shore hardness and water contact angle decrease. The elastic modulus of the coating is most sensitive to seawater immersion when 6% curing agent is added. The stability of organosilicon coating containing 5% curing agent and 8% silane coupling agent decreased obviously in seawater. Too much addition of curing agent will lead to the increase of the crosslinking degree of the silica chain, the densification of the network structure, and the increase of the hardness of the coating, which is not conducive to the antifouling performance of the coating; too much addition of silane coupling agent will lead to the increase of the hydrophilic groups on the coating surface, the decrease of the water contact angle, and the increase of the attachment of marine bacteria.
Key words: silicone; curing agent; silane coupling agent; performance
Liu Fujie, Qi Yuhong, Zhang Zhanping (Shipbuilding Engineering Laboratory of Dalian Maritime University, Dalian 116026, Liaoning)
Chinese Library Classification No.: tg174.46; tq317 document identification code: a Article No.: 1007-9289 (2014) 01-0114-06
0. Introduction
In recent years, the development and utilization of marine environmental resources have been expanding, and more and more attention has been paid to fuel consumption, greenhouse gas emissions, structural corrosion and damage caused by the fouling of marine organisms [1]. The application of antifouling coating on the surface of large marine structures, such as ships and offshore oil platforms, has long been an important way to solve the problem of fouling caused by the attachment of marine organisms, which is not only economical and efficient, but also the only way widely used [2]. However, the use of some toxic agents in antifouling coating also causes serious pollution of the marine environment [3-5]. To this end, with the long-term efforts of the international maritime organization, the use of organotin antifouling agent in antifouling paint has been completely banned worldwide in 2008 [6].
Low surface energy antifouling coating depends on its surface with low free energy, which makes it difficult to wet, spread and adhere the slime of marine organisms on the coating surface. Marine organisms are separated from the coating surface by peeling, plane shearing and non plane shearing, among which the energy required for peeling is the least [2]. The silicone low surface energy coating has a lower elastic modulus, and the dirt can be peeled off from the coating by the way of the least external force required. Even if the adhesion is not firm, it can easily fall off under the action of water flow. In addition, the surface of low surface energy antifouling coating is smooth, and compared with other antifouling coatings, it also has obvious drag reduction and consumption reduction effect [7]. Therefore, in recent years, it has become a research hotspot of non-toxic antifouling coatings at home and abroad.
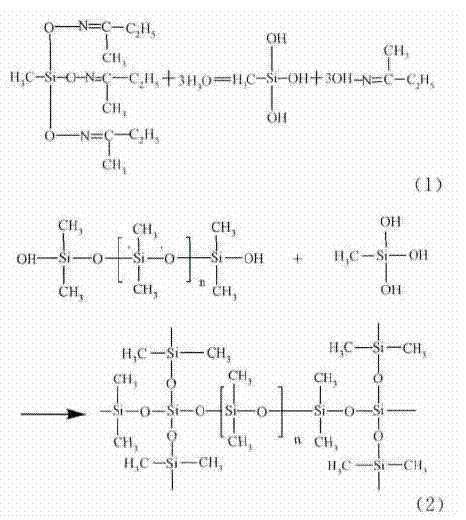
The curing mechanism of the silicone coating is that the siloxane chain is linked into a network structure through the crosslinking polycondensation reaction. The structure and amount of coating components directly determine and affect the structure and performance of silicone coating. At present, the low surface energy organosilicon antifouling coatings, which are widely used and researched in the market, are mainly crosslinked polycondensation system of PDMS and TEOS.
The reason why this kind of low surface energy organic silicon coating has not been widely used in production is mainly due to the following deficiencies: ① poor adhesion and poor repainting performance with the base material, the special organic silicon connecting paint is usually applied between the marine epoxy primer and the low surface energy organic silicon coating, the coating supporting system is complex and not easy to construct; ② low strength and easy to damage; ③ It is only applicable to ships with high navigation rate and high-speed operation [8]. It is easy to produce attached pollution at low speed and in the period of suspension, so it needs to be decontaminated regularly.
In order to improve the adhesion of low surface energy silicone coating and marine epoxy primer, reduce the complexity of the coating system, and reduce the construction difficulty, and improve the strength and durability of the coating, methyl tributyloxime silane (d31) was selected as the curing agent to improve the strength and shorten the curing time of the silicone coating; Previous studies have shown that the adhesive strength of silane coupling agent containing amino group and epoxy group is high [9]. In this paper, N - (β - aminoethyl) - γ - aminopropyltrimethoxysilane (kh792) is selected as silane coupling agent to improve the adhesion between silicone coating and epoxy primer; The effect of curing agent and silane coupling agent content on the properties of silicone coating was studied in order to provide the experimental basis for the development of practical silicone low surface energy antifouling coating which can be directly applied on marine epoxy primer.
1. Test materials and methods
1.1 test plan
The effects of curing agent and silane coupling agent on the properties of coatings were studied by single factor test. The coating is a two-component system, a component is composed of silicone resin, silane coupling agent, pigment and filler, and auxiliary agent; B component is composed of crosslinking curing agent d31 and toluene solution of dibutyltin dilaurate (dbtdl). According to the results of Xie Guoxian et al. [9] aimed at improving the adhesion between epoxy coating and steel substrate materials, it was found that the maximum adhesion could be obtained by adding 2% aminosilane coupling agent into epoxy coating. Referring to the research results of Xie Guoxian and other literatures, the range of the amount of curing agent and silane coupling agent was determined. The test is divided into two parts. The first part: fix the mass fraction of silane coupling agent in component A as 5%, and adjust the mass fraction of curing agent in component B as 3% and 4%, respectively